We use cookies to make your experience better.
TimmersGems has a new website, existing customers also need to register again.
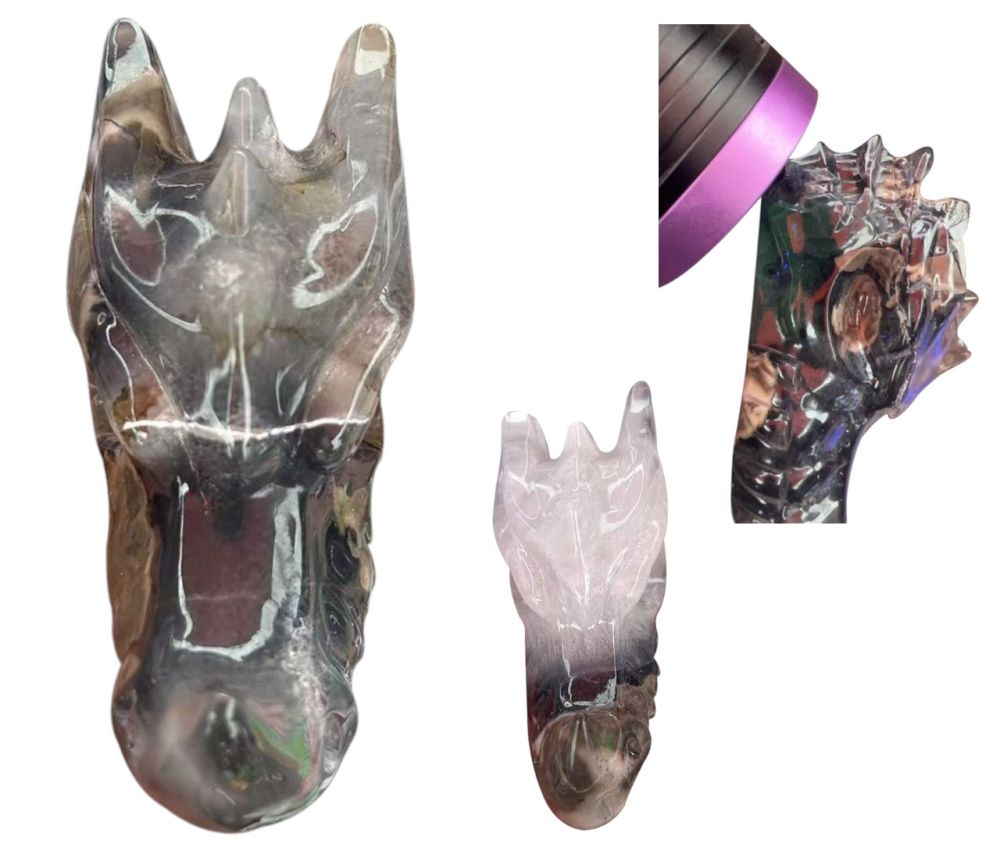
Dragon skull made of fluorescent agate (glows under UV light) from China.
New discovery: "Lumimous" agate with occasional fluorite spots recently discovered in China, including skulls. The stone could even be fluorescent.
Availability:
In stock
SKU
121782
Fluorescence is a unique type of luminescence. It concerns a physical phenomenon in which an atom or molecule absorbs a high-energy photon, leading to an electron in an excited state, which then falls back to the ground state. Radiation is emitted, which, due to relaxation and thermal losses, generally has lower energy than the high-energy photon. This makes it possible to convert invisible UV light into visible light. For example: UV light, a high-energy photon, is absorbed (absorption), after which green light can be emitted through fluorescence. (emissie). The concept of fluorescence originates from fluorite, a mineral that consists of the salt calcium fluoride (CaF2), a well-known substance that fluoresces.
| Dimensions | Divers |
|---|---|
| Country of Manufacture | China |